 Official websites use .gov
A .gov website belongs to an official government organization in the United States. Official websites use .gov
A .gov website belongs to an official government organization in the United States. |
 Secure .gov websites use HTTPS
A lock or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites. Secure .gov websites use HTTPS
A lock or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites. |
Solutions today for reefs tomorrow
Black band disease (BBD) is an infectious coral disease that is globally present and affects 19 scleractinian coral species. Black band disease is characterized by a tissue loss lesion demarcated by a black band, which spreads across a coral colony ultimately resulting in its death. Black band disease is one of the few coral diseases with a well-defined etiological cause, which has been identified as the sulfur-reducing activities of the bacteria that dominate the microbial consortium associated with the disease lesion.
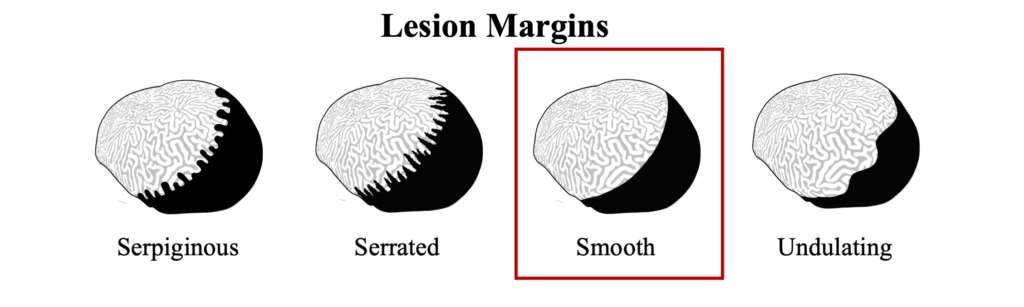
Black band disease is a tissue loss disease with a sharply demarcated line of healthy pigmented tissue next to the exposed skeleton of where tissue was recently lost. The margin of tissue and skeleton is covered with a darkly colored band created by a thick microbial mat, appearing either as black or dark red, depending on light conditions, that can vary in width from 1mm to several centimeters. Removing the microbial mat will reveal tissue that has been degraded into a gelatinous consistency that retains its healthy pigment.
Black band disease lesions often begin as a focal point at the apical or peripheral portions of the coral that spreads outward at a rate of 1mm-1cm/day to completely encircle the colony. The width of the dark band that defines the advancing lesion remains consistent throughout disease progression.
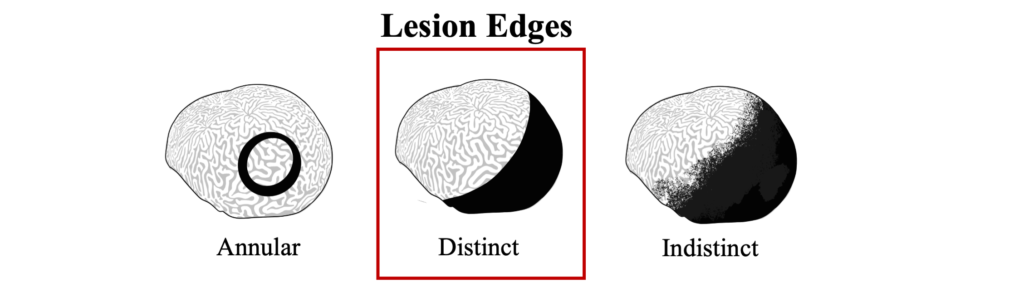
The distinct margin of tissue loss on one the following side of the progressing microbial mat is what differentiates BBD from ciliate infections that can have a similar dark coloration near a tissue loss lesions, but which appear to be more indistinct.
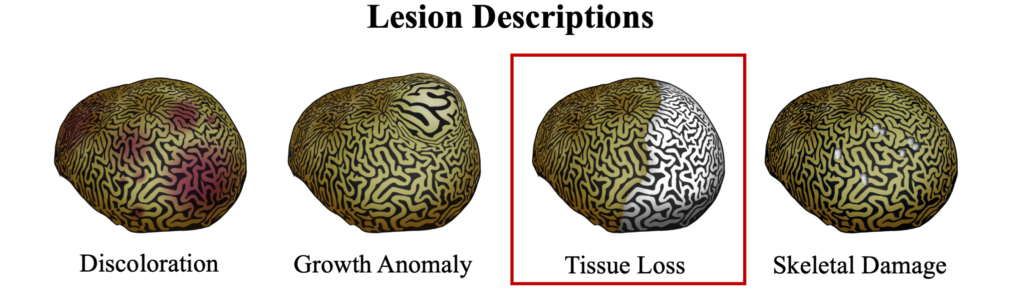
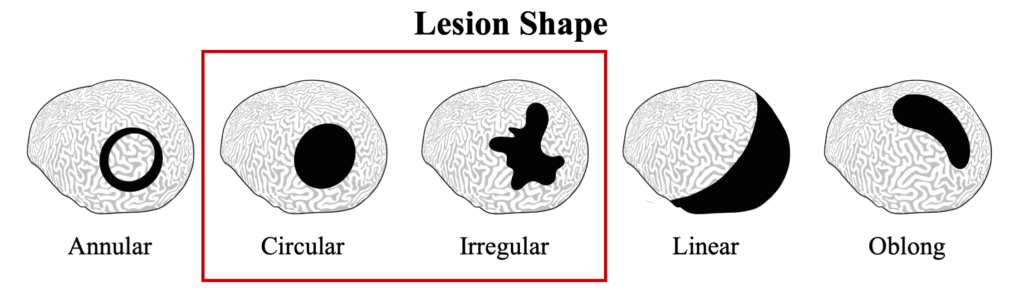
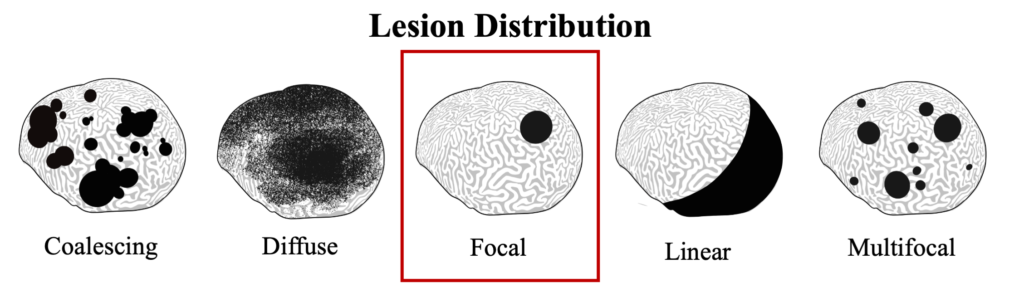
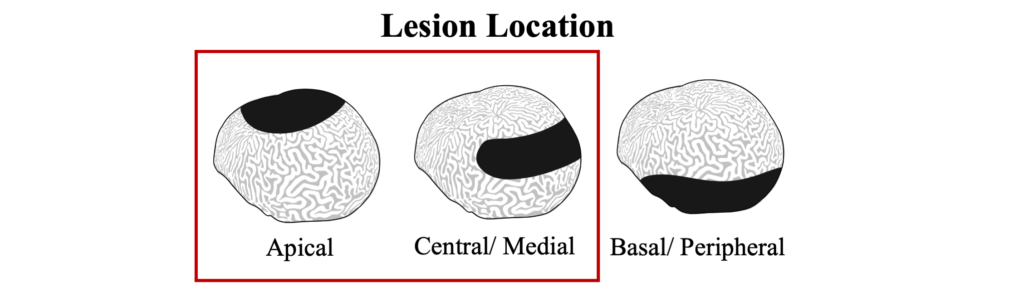
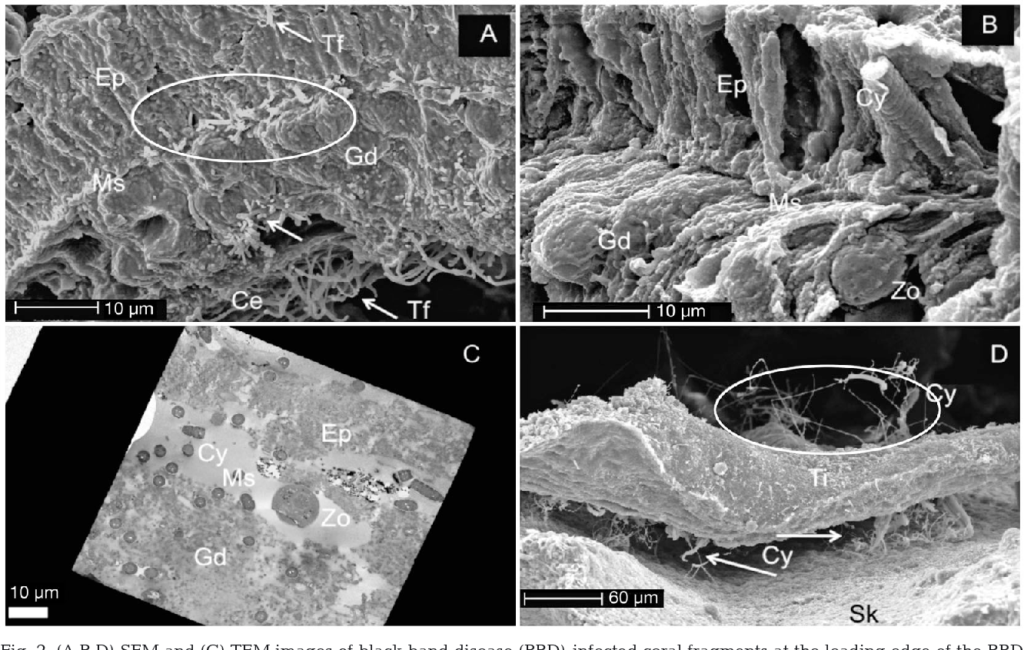
Black Band Disease is associated with a polymicrobial consortium that together generates anoxic conditions and toxic compounds that lyse coral tissues. This consortium generates a sulfur biochemical cycle that causes an anoxic, sulfur-rich micoenvironment against the underlying coral tissue, similar to cyanobacterial mats that would be found at hot springs and hypersaline lagoons. The four main components of this consortium are cyanobacteria (or “phototrophs”), sulfur-reducing bacteria, sulfur-oxidizing bacteria, and heterotrophs. Of these groups, only the community of cyanobacteria and sulfur-reducing bacteria are known to be directly involved in BBD pathobiology.
Cyanobacteria are the most dominant taxa in the BBD microbial consortium and are what gives the characteristic microbial mat its dark color, due to their high concentration of the pigment phycoerythrin. There are 10 known strains of cyanobacteria in culture that are associated with BBD, the most common being the species Roseofilum reptotaenium. The cyanobacteria present in the BBD lesion all produce the toxin microcystin, which is toxic to coral tissues through its ability to disrupt cell membrane potential and initiate apoptosis.
The diverse group of sulfate-reducing bacteria, members of the group delta-proeobacteria, contribute to BBD pathology along with cyanobacteria by producing sulfides, which are toxic to corals. Sulfides are produced as a byproduct of their anaerobic respiration, which uses sulfate sourced from the surrounding seawater as the terminal electron acceptor in their metabolism. Limited diffusion within the microbial mat causes sulfides to build up, making this taxa crucial to the chemical microenvironment that defines BBD. While this accumulation of sulfide has been shown to be required for BBD infection onset, it does not appear to be required for the lesion to persist over time, so other unknown toxins are involved in BBD pathology.
Sulfate-oxidizing bacteria are the second most prominent taxa in BBD lesions in terms of biomass, after cyanobacteria. To date, three genera have been detected in BBD samples: Beggiata, Thiomicrospira, andArcobacter, all of which are members of gama-proteobacteria. This group lives at the oxygen-sulfate interface that migrates through the center of the 1mm thick mat, which delineates the anoxic, sulfur-rich region near the colony surface and the oxygenated layer above.
The consortium of heterotrophic bacteria within the BBD microbial mat is highly diverse and the roles it might play in BBD pathology are not well understood. The group is dominated by members of alpha-proteobacteria, and the most widely detected species in BBD samples is Roseovarius crassostrear, which is a known pathogen in diseases of oysters.
The tissue loss that is characteristic of BBD is caused both by the toxins mentioned above (sulfide produced by sulfer-reducing bacteria; microcystin produced by cyanobacteria) and by mechanical damage to the tissue as the cyanobacteria migrate through the coral’s mesoglea (the layer of connective tissue between the epidermis and gastrodermis), causing it to separate from the underlying tissue and lose its structural integrity.
Black band disease has been observed to infect 19 species of scleractinan coral and six species of soft corals. Interestingly, while a broad range of coral hosts are reportedly susceptible, BBD infections are often only present on a single species within a reef community at a time, with other susceptible species showing no reaction. Prevalence of BBD typically remains below 1% and is only present in the warmer summer months when water temperatures rise above 27.5C.
The precise mode of transmission on a reef is not currently known, however, transmission and epidemiological modeling studies have shown that it can be transferred between colonies via vectors (such as fish), direct contact, inoculation with the microbial mat, and via water borne particulates.
While the reef-wide prevalence of BBD is typically low, the tendancy for the infection to target large reef-building species and remove their tissue at a rate that far exceeds their growth makes BBD a formidable threat to the ecological structure and landscape of Caribbean reef communities. BBD can be treated in the field by immobilizing the microbial mat at its lesion, either by manual removal or by suffocating the photosynthesizing consortium by smothering the mat in an opaque substance.
Barneah, O., Ben-Dov, E., Kramarsky-Winter, E., & Kushmaro, A. (2007). Characterization of black band disease in Red Sea stony corals. Environmental Microbiology, 9(8). https://doi.org/10.1111/j.1462-2920.2007.01315.x
Bruckner, A. W., Bruckner, R. J., & Williams, E. H. (1997). Spread of a black-band disease epizootic through the coral reef system in St. Ann’s Bay, Jamaica. Bulletin of Marine Science, 61(3).
Carlton, R. G., & Richardson, L. L. (1995). Oxygen and sulfide dynamics in a horizontally migrating cyanobacterial mat: Black band disease of corals. FEMS Microbiology Ecology, 18(2). https://doi.org/10.1016/0168-6496(95)00052-C
Casamatta, D., Tanić, D. S., Gantar, M., & Richardson, L. L. (2012). Characterization of Roseofilum reptotaenium (Oscillatoriales, Cyanobacteria) gen. et sp. nov. isolated from Caribbean Black band disease. Phycologia, 51(5). https://doi.org/10.2216/11-10.1
Cooney, R. P., Pantos, O., le Tissier, M. D. A., Barer, M. R., O’Donnell, A. G., & Bythell, J. C. (2002). Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Environmental Microbiology, 4(7). https://doi.org/10.1046/j.1462-2920.2002.00308.x
Edmunds, P. J. (1991). Extent and effect of Black Band Disease on a Caribbean reef. Coral Reefs, 10(3). https://doi.org/10.1007/BF00572175
Edmunds, P. J. (2000). Recruitment of scleractinians onto the skeletons of corals killed by black band disease. Coral Reefs, 19(1). https://doi.org/10.1007/s003380050229
Frias-Lopez, J., Bonheyo, G. T., Jin, Q., & Fouke, B. W. (2003). Cyanobacteria associated with coral black band disease in Caribbean and Indo-Pacific reefs. Applied and Environmental Microbiology, 69(4). https://doi.org/10.1128/AEM.69.4.2409-2413.2003
Frias-Lopez, J., Klaus, J. S., Bonheyo, G. T., & Fouke, B. W. (2004). Bacterial community associated with black band disease in corals. Applied and Environmental Microbiology, 70(10). https://doi.org/10.1128/AEM.70.10.5955-5962.2004
Garrett, P., & Ducklow, H. (1975). Coral diseases in Bermuda. Nature, 253(5490). https://doi.org/10.1038/253349a0
Glas, M. S., Sato, Y., Ulstrup, K. E., & Bourne, D. G. (2012). Biogeochemical conditions determine virulence of black band disease in corals. ISME Journal, 6(8). https://doi.org/10.1038/ismej.2012.2
Kaczmarsky, L. T., Draud, M., & Williams, E. H. (2005). Is there a relationship between proximity to sewage effluent and the prevalence of coral disease? Caribbean Journal of Science, 41(1).
Kuta, K. G., & Richardson, L. L. (1996). Abundance and distribution of black band disease on coral reefs in the northern Florida Keys. Coral Reefs, 15(4). https://doi.org/10.1007/s003380050046
MacKintosh, C., Beattie, K. A., Klumpp, S., Cohen, P., & Codd, G. A. (1990). Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Letters, 264(2). https://doi.org/10.1016/0014-5793(90)80245-E
Miller, A. W., & Richardson, L. L. (2011). A meta-analysis of 16S rRNA gene clone libraries from the polymicrobial black band disease of corals. FEMS Microbiology Ecology, 75(2). https://doi.org/10.1111/j.1574-6941.2010.00991.x
Miller, A. W., & Richardson, L. L. (2012). Fine structure analysis of black band disease (BBD) infected coral and coral exposed to the BBD toxins microcystin and sulfide. Journal of Invertebrate Pathology, 109(1). https://doi.org/10.1016/j.jip.2011.09.007
Myers, J. L., & Richardson, L. L. (2009). Adaptation of cyanobacteria to the sulfide-rich microenvironment of black band disease of coral. FEMS Microbiology Ecology, 67(2). https://doi.org/10.1111/j.1574-6941.2008.00619.x
Myers, J. L., Sekar, R., & Richardson, L. L. (2007). Molecular detection and ecological significance of the cyanobacterial genera Geitlerinema and Leptolyngbya in black band disease of corals. Applied and Environmental Microbiology, 73(16). https://doi.org/10.1128/AEM.00900-07
Richardson, L. L., Miller, A. W., Broderick, E., Kaczmarsky, L., Gantar, M., Stanić, D., & Sekar, R. (2009). Sulfide, microcystin, and the etiology of black band disease. Diseases of Aquatic Organisms, 87(1–2). https://doi.org/10.3354/dao02083
Richardson, L. L., Sekar, R., Myers, J. L., Gantar, M., Voss, J. D., Kaczmarsky, L., Remily, E. R., Boyer, G. L., & Zimba, P. v. (2007). The presence of the cyanobacterial toxin microcystin in black band disease of corals. FEMS Microbiology Letters, 272(2). https://doi.org/10.1111/j.1574-6968.2007.00751.x
Rodríguez, S., & Cróquer, A. (2008). Dynamics of Black Band Disease in a Diploria strigosa population subjected to annual upwelling on the northeastern coast of Venezuela. Coral Reefs, 27(2). https://doi.org/10.1007/s00338-007-0341-8
Rützler, K., & Santavy, D. L. (1983). The Black Band Disease of Atlantic Reef Corals: I. Description of the Cyanophyte Pathogen. Marine Ecology, 4(4). https://doi.org/10.1111/j.1439-0485.1983.tb00116.x
Sato, Y., Bourne, D. G., & Willis, B. L. (2009). Dynamics of seasonal outbreaks of black band disease in an assemblage of Montipora species at Pelorus Island (Great Barrier Reef, Australia). Proceedings of the Royal Society B: Biological Sciences, 276(1668). https://doi.org/10.1098/rspb.2009.0481
Sato, Y., Bourne, D. G., & Willis, B. L. (2011). Effects of temperature and light on the progression of black band disease on the reef coral, Montipora hispida. Coral Reefs, 30(3). https://doi.org/10.1007/s00338-011-0751-5
Sato, Y., Willis, B. L., & Bourne, D. G. (2010). Successional changes in bacterial communities during the development of black band disease on the reef coral, Montipora hispida. ISME Journal, 4(2). https://doi.org/10.1038/ismej.2009.103
Sekar, R., Kaczmarsky, L. T., & Richardson, L. L. (2008). Microbial community composition of black band disease on the coral host Siderastrea siderea from three regions of the wider Caribbean. Marine Ecology Progress Series, 362. https://doi.org/10.3354/meps07496
Sekar, R., Mills, D. E. K., Remily, E. R., Voss, J. D., & Richardson, L. L. (2006). Microbial communities in the surface mucopolysaccharide layer and the black band microbial mat of black band-diseased Siderastrea siderea. Applied and Environmental Microbiology, 72(9). https://doi.org/10.1128/AEM.00843-06
Stal, L. J. (1995). Physiological ecology of cyanobacteria in microbial mats and other communities. New Phytologist, 131(1). https://doi.org/10.1111/j.1469-8137.1995.tb03051.x
Stanić, D., Oehrle, S., Gantar, M., & Richardson, L. L. (2011). Microcystin production and ecological physiology of Caribbean black band disease cyanobacteria. Environmental Microbiology, 13(4). https://doi.org/10.1111/j.1462-2920.2010.02388.x
Voss, J. D., & Richardson, L. L. (2006). Nutrient enrichment enhances black band disease progression in corals. Coral Reefs, 25(4). https://doi.org/10.1007/s00338-006-0131-8
Zvuloni, A., Artzy-Randrup, Y., Stone, L., Kramarsky-Winter, E., Barkan, R., & Loya, Y. (2009). Spatio-temporal transmission patterns of black-band disease in a coral community. PLoS ONE, 4(4). https://doi.org/10.1371/journal.pone.0004993